Scientific sessions of the American Diabetes Association (ADA) 2023 in San Diego, USA
June 23-26 2023
CeeD was in San Diego for the major scientific event of the American Diabetes Association.
With Dr Karim Bouzakri, our laboratory director:
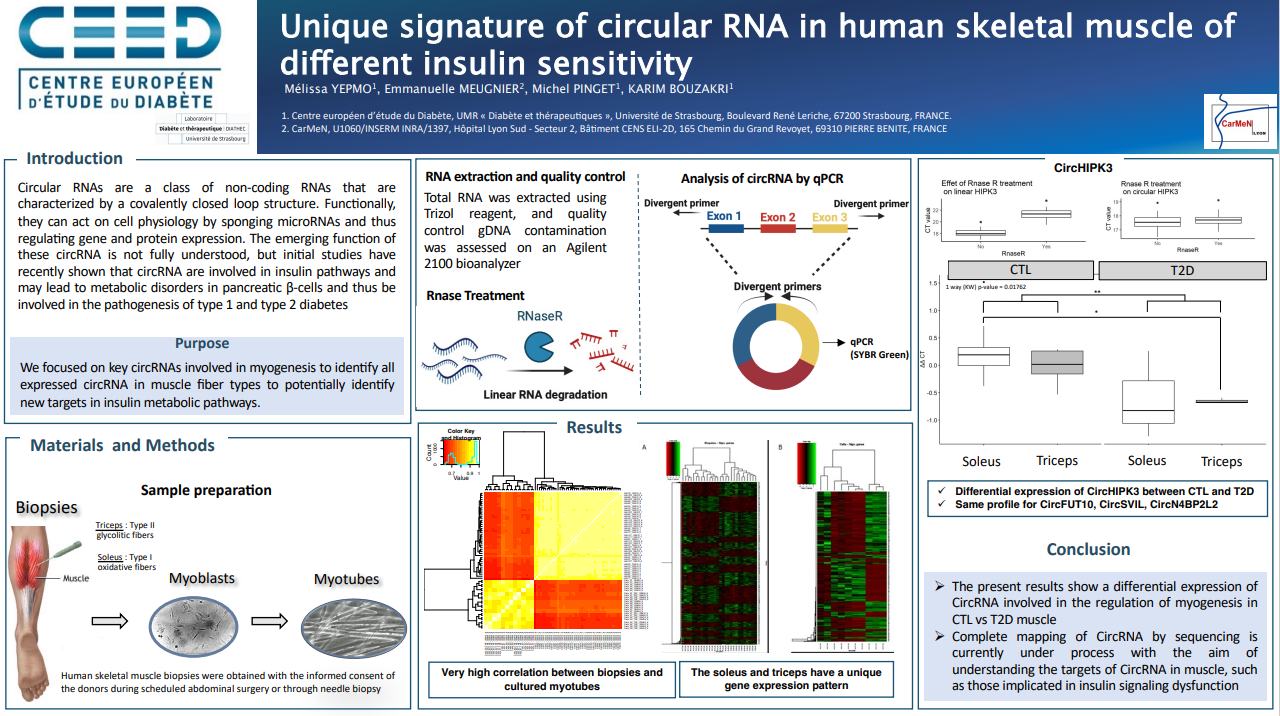
1 - Mélissa Yepmo explained her PhD research on CircRNAs, an emergent class of non coding RNA as new potentiel therapeutic target in skeletal muscle and their role in insulin secretion dysfunction
Abstract :
"Diabetes pathologies are complex, affecting numerous organs such as liver, pancreas, and skeletal muscle. Circular RNAs (circRNA) are a class of non-coding RNAs that are characterized by a covalently closed loop structure. Functionally, they can act on cell physiology by sponging microRNAs and thus regulating gene and protein expression. The emerging function of these circRNA is not fully understood, but initial studies have recently shown that circRNA are involved in insulin secretion regulation. Therefore, deregulation of this class of RNAs may lead to metabolic disorders in pancreatic β-cells and thus be involved in the pathogenesis of type 1 (T1DM) and type 2 diabetes (T2DM). Our research is focused on the unique signature by circular RNA and the impact on skeletal muscle metabolism. We demonstrated that human skeletal muscle cells in culture maintain their gene expression phenotype. A di"erent gene signature of the cells according to their di"erent muscular fiber type sources (oxidative versus glycolytic versus mixt) was confirmed. Moreover, an independent microRNA composition has also been highlighted. As CircRNA can regulate microRNAs and then gene expression and proteins, we wondered whether these observed signatures could be explained by circRNA. We therefore focused on key circRNAs involved in myogenesis (such as circZNF609, circHIPK3) and we were able to see that there is a difference in expression of circRNA according to the muscle type. Moreover, we analyzed correlations between circRNA levels and insulin sensitivity (measured by PKB phosphorylation). Finally, we are currently completing our analysis with in-depth sequencing techniques to identify all expressed circRNA in muscle fiber types to potentially identify new targets in insulin metabolic pathways."
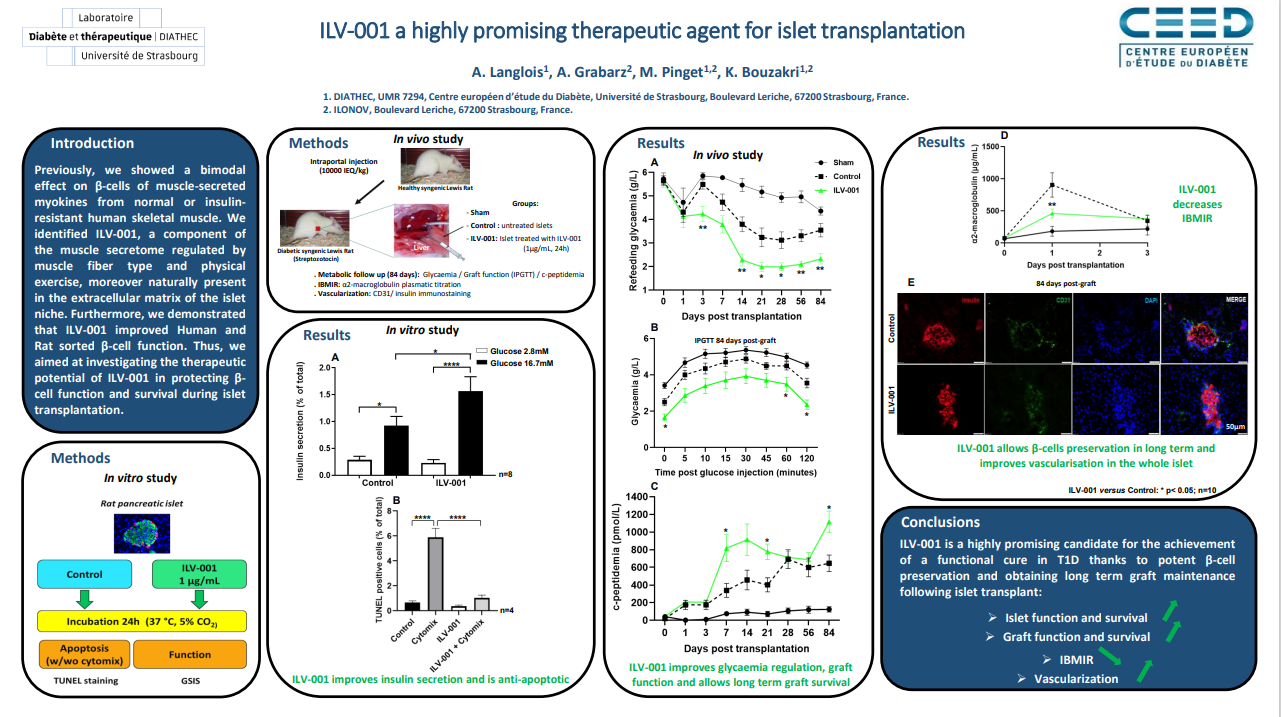
2 - Dr Allan LANGLOIS presented the project untitled “ILV-001 a highly promising therapeutic agent for islet transplantation” (1494-P, session “Beta cells replacement”).
In this work, we aimed at investigating the therapeutic potential of ILV-001, a component of the muscle secretome regulated by physical exercise and naturally present in the extracellular matrix of the islet niche, in protecting β-cell function and survival during islet transplantation.
Abstract :
ILV-001 a highly promising therapeutic agent for islet transplantation
A. Langlois 1, A. Grabarz2, M. Pinget1,2, K. Bouzakri1,2
1. DIATHEC, UMR 7294, Centre européen d’étude du Diabète, Université de Strasbourg, Boulevard Leriche, 67200 Strasbourg, France.
2. ILONOV, Boulevard Leriche, 67200 Strasbourg, France.
Introduction:
Our team has shown a bimodal effect on β-cells of muscle-secreted myokines from normal or insulin-resistant human skeletal muscle. We identified ILV-001, a component of the muscle secretome regulated by muscle fiber type and physical exercise, moreover naturally present in the extracellular matrix of the islet niche. Thus, we aimed at investigating the therapeutic potential of ILV-001 in protecting β-cell function and survival during islet transplantation.
Methods:
In vitro: Rat islets were incubated 24h ± ILV-001 (1µg/mL). Islet survival was assessed by TUNEL assay ± cytomix. Islet function was evaluated using GSIS test.
In vivo: 10000 IEQ/kg ± ILV-001 were injected intraportaly in diabetic syngeneic rats. For 3 months body weight gain, glycaemia, c-peptidemia and graft function (IPGTT) were followed. Inflammation was evaluated titrating plasmatic α2-macroglobulin. Histology was performed on grafted islets.
Results:
In vitro: ILV-001 improves islet function and survival and potentiates insulin secretion after GSIS test.
In vivo: ILV-001 improves graft function in terms of glycaemia (p<0.001) and c-peptidaemia (p<0.01) and decreases exogenous insulin intake requirements. ILV-001 reduced inflammation 24h post graft with a lower plasmatic α2-macroglobulin versus control with respectively 459±70 versus 903±191 µg/mL (p<0.05). Finally, histology showed a preservation of insulin positive cells with ILV-001 versus control at the end of study (69±3% versus 39±3% of β-cells/islet, p< 0.001).
Conclusion:
ILV-001 is a highly promising candidate for the achievement of a functional cure in T1D thanks to potent β-cell preservation and obtaining long term graft maintenance following islet transplant.